Mechanobiology
- Light-Sheet Fluorescent Microscope
- Endothelial Function
- Oxidative Stress
- Computational Fluid Dynamics
- Cardiac Morphogenesis
- Vascular Repair
- Cardiac Trabeculation
- Wirelessly Powered Micro-Actuators for Intravascular Deployment
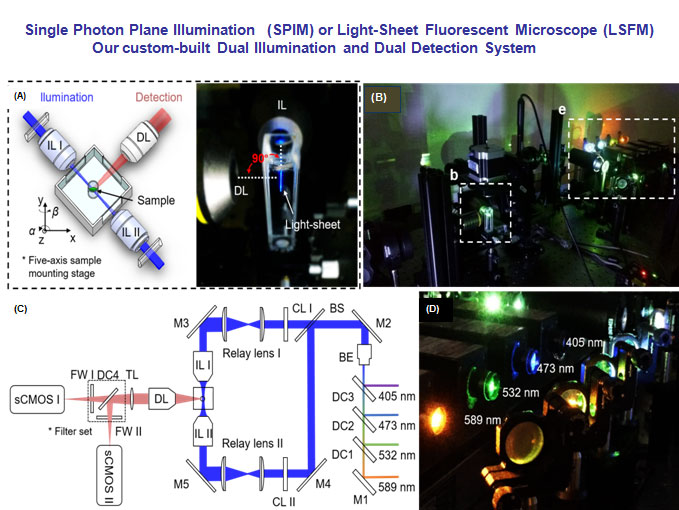
(A) The specimen is mounted at the intersection of the illumination lens (IL) with the detection lens (DL). The laser light-sheet is excited from the IL in a 2-D plane which is orthogonal to the detection axis. The SPIM system provides a long working distance with air objective lenses in comparison to water-dipping lenses in conventional light-sheet systems. (B-C) A photo and a schematic illustrate the layout of the light-sheeting imaging system. A cylindrical lens (CL) converts the laser beam to a sheet of laser light that can transversely illuminate a thin layer of the sample. The illuminated 2-D thin layer (fluorescent detection in red) is captured by the high-frame rate sCMOS camera. The filter wheels (FW I and II) in front of sCMOS cameras are used for multi-color acquisitions. (D) A photo depicts an array of laser beams aligned for multi-channel fluorescent detection. M: mirror; BS: beam splitter; BE: beam expander; TL: tube lens; DC: dichroic mirror; FW: filter wheel.
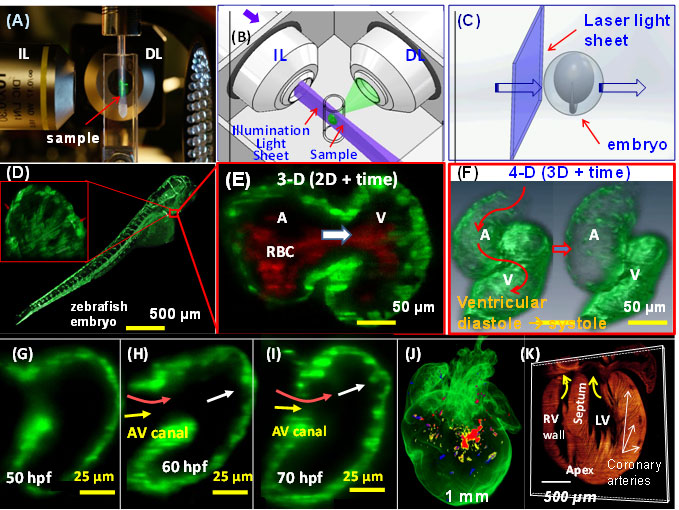
4-D LSFM imaging (3-D + time) of zebrafish. (A) The sample is placed at the orthogonal intersection between the illumination lens (IL) and detection lens (DL) in the SPIM system. (B) A cylindrical lens generates a thin laser light-sheet, and the IL lens excites a thin slice of the sample in a 2-D plane. The fluorescence from the illuminated planes is orthogonally detected by the DL. (C) A schematic diagram
illustrates the laser light-sheet sectioning across a zebrafish embryo. (D) The entire embryo was imaged within 30 sec at the single cellular resolution. Inset reveals the trabecular endocardium. (E) Magnification of the heart reveals the contracting fli-1-eGFP-labeled myocardium and flowing DsRed-labeled red blood cells across the AV valve. (F) The integration of SPIM image with synchronization algorithm reveals
a 4-D heart. (G) Trabecular ridges were absent in the myocardium at ~50 hpf. (H) Ridges protruding into the ventricular cavity occurred at ~60 hpf near the myocardial lining experienced pulsatile blood inflow. (I) Distinct trabecular ridges occurred at ~70 hpf. (J) 3-D LSFM tracks the multi-channel fluorescently labeled cardiac progenitor lineage in an E7 neonatal rainbow mouse heart. (j) 3-D Td tomato-labeled cardiomyocytes reveals the structure in the E7 heart. The yellow arrows point to the atrio-ventricular valves.

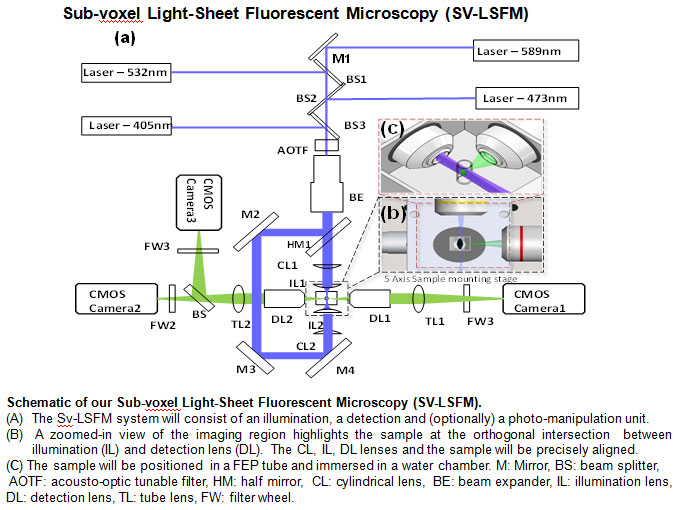
Single illumination LSFM imaging. (a) The sample is positioned at the orthogonal
intersection of the illumination lens (IL) and detection lens (DL). (b) A laser light-sheet
is applied to rapidly illuminate the sample. The illuminated planes are orthogonally
detected by the detection objective (DL). (c) A sheet of light transverses the embryo.
(d) The entire 3-D embryo can be imaged. Inset reveals the 3-D image of red blood cells (RBC)
across the AV valve. (e) 3-D LSFM tracks the multi-channel fluorescent images of the
cardiac progenitor lineage in an E7 neonatal rainbow mouse heart. (f) 3-D Td tomato-labeled
cardiomyocytes show the myocardium of the E7 heart. The yellow arrows point to the
atrio-ventricular valves. (g) 3-D honey-comb like hydrogel. (h) 3-D LSFM tracks the
multi-channel fluorescent images of adult mouse ocular anatomy. (i) 3-D adult mouse
retinal vasculature. (i) Retinal vasculature. (k) Vascular calcification in an adult mouse
aortic arch.

Single illumination LSFM imaging. (a) The sample is positioned at the orthogonal
intersection of the illumination lens (IL) and detection lens (DL). (b) A laser light-sheet
is applied to rapidly illuminate the sample. The illuminated planes are orthogonally
detected by the detection objective (DL). (c) A sheet of light transverses the embryo.
(d) The entire 3-D embryo can be imaged. Inset reveals the 3-D image of red blood cells (RBC)
across the AV valve. (e) 3-D LSFM tracks the multi-channel fluorescent images of the
cardiac progenitor lineage in an E7 neonatal rainbow mouse heart. (f) 3-D Td tomato-labeled
cardiomyocytes show the myocardium of the E7 heart. The yellow arrows point to the
atrio-ventricular valves. (g) 3-D honey-comb like hydrogel. (h) 3-D LSFM tracks the
multi-channel fluorescent images of adult mouse ocular anatomy. (i) 3-D adult mouse
retinal vasculature. (i) Retinal vasculature. (k) Vascular calcification in an adult mouse
aortic arch.

Fluid shear stress imparts both metabolic and mechanical effects on vascular endothelial function. A complex flow profile develops at the arterial bifurcations, namely, flow separation and migrating stagnation points, creating low and oscillating shear stress (Fig. 1). At the lateral walls of bifurcations, disturbed flow, including oscillatory flow (bidirectional and axially misaligned flow), is considered to be an inducer of oxidative stress, favoring the initiation of atherosclerosis, whereas in the medial wall of bifurcation, pulsatile flow (unidirectional and axially aligned flow) down-regulates inflammatory cytokines, adhesion molecules, and oxidative stress.
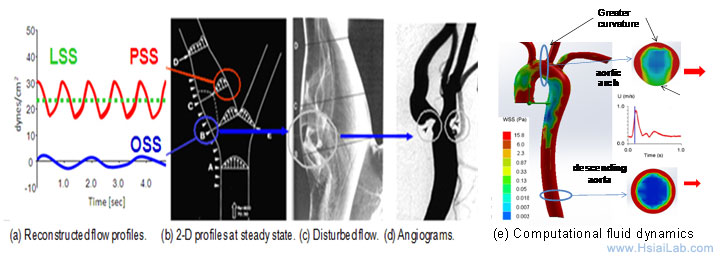
Fig. 1. Mechanotransduction underlying vascular metabolic and inflammatory responses. (a) Shear stress profiles at the lateral and medial walls of arterial bifurcations. (b) PSS occurs at the medial wall (red circle), whereas OSS occurs at the migrating stagnation point of the lateral wall (blue circle). (c) Flow separation and disturbed flow develop at the lateral wall. (d) Angiogram supports the predilection sites for atherosclerosis. (e) Computational fluid dynamic simulation of aortic arch, thoracic aorta and descending aorta at an instantaneous moment during a cardiac cycle.
1. Hsiai* T, Cho S. K., Reddy S, Hama S., Navab M., Demer L., Honda H, Ho, C. M. Pulsatile flow patterns regulate monocyte adhesion to endothelial cells in response to oxidized lipids. Arterioscler Thromb Vasc Biol . 2001, 21(11): 1770-1776.
2. Hsiai* T, Cho S. K, Honda, H., Hama S, Navab M, Demer L., Ho C. M. Endothelial cell dynamics under pulsating flow: Significance of high- vs. low shear stress slew rates. Annals of Biomedical Engineering, 2002,30(5):646-656
3. Hsiai*, T. Ing, M., Cho S. K., Wong, P., Hama S., Navab M, Demer, L., Ho, C., Monocyte recruitment to endothelial cells In response to oscillatory shear stress. The FASEB Journal, 2003, Vol. 17:1648-1657
4. Hwang, J., Ing, M., Salazar, A., Navab, M. Sevanian, A., Hsiai*, T. Pulsatile vs. oscillatory flow regulates NADPH Oxidase Subunit: Implication for Native LDL Oxidation. Circulation Research, 2003; 93:1225-1232.
5. Hsiai*, T. Salazar, A., Cho, S. K., Wang, P. K., Navab, M., Micro Sensors: Linking Inflammatory Responses with Oscillatory Shear Stress. Annals of Biomedical Engineering, 2004, 32(2):189-201.
6. Hsiai*, T., Lin, C., Rouhanizadeh, M, S., Cadenas, E., Hwang, J., Hazen, S. L., Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free Radic Bio Med. 2007 Feb 15;42(4):519-29.
Our study revealed changes in hemodynamics and development of lesions after 8 weeks of high-fat diet. Our MEMS sensors were deployed to assess ISS in real-time (Fig. 2). Experimental ISS measurements (red) overlapped closely with those of computational fluid dynamic codes (blue) in various segments of rabbit aorta (Fig. 2a). Deployment of IVUS revealed intimal hyperplasia in relation to changes in ISS analysis (Fig. 2). High-frequency IVUS demonstrate cross sections of descending aortic arch, thoracic, and abdominal aortas with a high spatial resolution (Figs. 2b-d). The time-averaged ISS after high-fat diets increased in regions of intimal hyperplasia; thus, supporting the basis to couple ISS with EIS signals (Fig. 2e). The reason for the increase in time-averaged ISS included intimal hyperplasia, rising serum viscosity from hypercholesterolemia (data not shown), and endoluminal remodeling. In Aim 2, we will deploy the high-frequency IVUS to guide the micro-shear stress sensors and concentric bipolar microelectrodes.
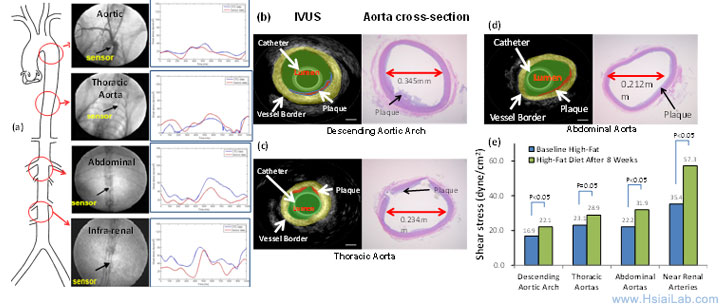
Fig. 2. Integrating real-time ISS, IVUS, and validation with histology to assess changes in intimal hyperplasia after 8 weeks of high-fat diet. (a) Real-time in vivo ISS profiles in various regions of aorta. The red curves denote baseline and blue curves post diet. (b) - (d) Comparisons of high-frequency IVUS with histopathology of endoluminal lesions reveals high-resolution images. (e) Time-averaged ISS signals are increased after 8 weeks of the high-fat diet.
7. Rouhanizadeh, M., Soundararajan, G., Ascara, D., Lo, R., Browand, F., Hsiai*, T., MEMS sensors to resolve spatial variations in shear stress in a 3-D bifurcation model, IEEE Sensors, February, 2006, 6(1):78-88.
8. Ai, L., Rouhanizadeh, M., Wu, J., Yi Chu Y., Miller, J., Heistad, D., Hsiai*, T., Oscillatory Shear Stress Influences Spatial Variations in Vascular MnSOD Expression and Nitrotyrosine Formation, Am J of Physiol: Cell Physiol. 2008 Jun;294(6):C1576-85.
9. M. Rouhanizadeh, W. Takabe, L. Ai, T. K. Hsiai*, Monitoring oxidative stress in vascular endothelial cells in response to fluid shear stress: from biochemical analyses to micro- and nanotechnologies.Methods Enzymol. 2008;441:111-50. Review.
10. H. Yu, L. Ai, M. Rouhanizadeh, D. Patel, E. S. Kim, T. K. Hsiai*. Flexible Polymer Sensors for In Vivo Intravascular Shear Stress Analysis, IEEE/ASME Journal of Micro Electro Mechanical Systems. 2008 October, Volume 17(5). 1178-1186.
11. Li, R, Rouhanizadeh, L. Ai, W. Takabe, T. K. Hsiai*, Shear Stress Regulates Mitochondrial Membrane Potential: Implication of MnSOD. Biochem Biophys Res Commun. 2009 Oct 16;388(2):406-12.
12. Takabe, W., Li, R., Yu F, Li A, Berliner, J., Hsiai* TK. Oxidized LDL-Activated JNK Regulates Mn-SOD Ubiquitination: Implication for Mitochondrial Redox Status and Apoptosis. Arteriosclerosis Thrombosis, and Vascular Biology. 2010;30:436-441.
13. Ai L, Zhang L, Dai W, Shung KK, Hsiai*, TK.. Real-time Assessment of Flow Reversal in an Eccentric Arterial Stenotic Model. J Biomechanics 2010 Oct 19;43(14):2678-83.
14. Yu F, Ai, L., Dai, W. Rozengurt N., Yu, H., Hsiai*, TK. MEMS Thermal Sensors to Detect Changes in Heat Transfer in the Pre-Atherosclerotic Regions of Fat-Fed New Zealand White Rabbits. Ann of Biomed Eng. 2011 Jun;39(6):1736-44. PMID:21380571
15. Fei Yu, Juhyun Lee, Nelson Jen, Xiang Li, Qiang Zhang, Rui Tang, Qifa Zhou, E.S. Kim, Tzung Hsiai*, Elevated Electrochemical Impedance in the Endoluminal Regions with High Shear Stress: Implication for Assessing Lipid-Rich Atherosclerotic Lesions. Biosensors and Bioelectronics. 2013 May 15;43:237-44
16. Nelson Jen, Fei Yu, Steve Wusmund, Mohamed Hamden, Tzung Hsiai*. Atrial Fibrillation Pacing Decreases Intravascular Shear Stress in a New Zealand White Rabbit Model: Implications in Endothelial Function. Biomechanics and Modeling in Mechanobiology. 2013 Jun;12(4):735-745.
Vascular oxidative stress and autophagic flux. Hemodynamic shear stress imparts both mechanical and metabolic effects on vascular endothelial function (Fig. 3). A complex flow profile develops at the arterial bifurcations, namely, flow separation and migrating stagnation points, creating low and oscillating shear stress (OSS) (Fig. 1). At the lateral wall of a bifurcation or lesser curvature, disturbed flow, including OSS (bidirectional and axially misaligned flow), is considered to be an inducer of oxidative stress, favoring the initiation of atherosclerosis, whereas in the medial wall of a bifurcation, greater curvature or the straight region, pulsatile shear stress (PSS: unidirectional and axially aligned flow) down-regulates pro-inflammatory mediators and oxidative stress.
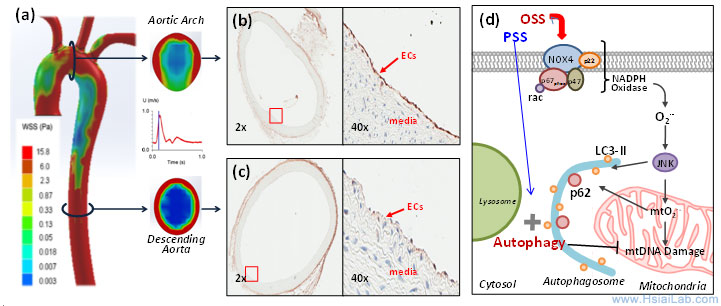
Fig. 3. Temporal ![]() and spatial
and spatial ![]() variations in shear stress. (a) The instantaneous wall shear stress is low in the lesser curvature of the aortic arch, but is circumferentially high in the descending aorta. (b) Immuno-staining reveals prominent p62 staining (arrow) in the lesser curvature where endothelial cells (ECs) were exposed to disturbed flow, including OSS. (c) p62 staining is nearly absent in the descending aorta where ECs were exposed to PSS. (d) OSS induces NADPH oxidase-mediated oxidative stress and c-Jun N-terminal Kinase (JNK) activation, leading to mitochondrial dysfunction. Sustained OSS impairs autophagic flux, leading to p62 accumulation, mtO2•- production, and mtDNA damage.)
variations in shear stress. (a) The instantaneous wall shear stress is low in the lesser curvature of the aortic arch, but is circumferentially high in the descending aorta. (b) Immuno-staining reveals prominent p62 staining (arrow) in the lesser curvature where endothelial cells (ECs) were exposed to disturbed flow, including OSS. (c) p62 staining is nearly absent in the descending aorta where ECs were exposed to PSS. (d) OSS induces NADPH oxidase-mediated oxidative stress and c-Jun N-terminal Kinase (JNK) activation, leading to mitochondrial dysfunction. Sustained OSS impairs autophagic flux, leading to p62 accumulation, mtO2•- production, and mtDNA damage.)
Autophagy is tightly regulated in the intracellular bulk degradation/recycling system for maintenance of cellular homeostasis. Flow-conditioned cells are more resistant to oxidant-induced cell injury, and this cytoprotective effect was abolished after inhibition of autophagy. Conversion of LC3-I (Atg8) to LC3-II allows the anchorage of Atg8/LC3 to the phagophore as an essential expansion to form autophagosomes.(10) Autophagy substrates are targeted for degradation by associating with p62/SQSTM1, a multi-domain protein that functions as a selective autophagy receptor, and deficiency in autophagy leads to intracellular accumulation of p62, a marker for an incomplete autophagy process or impaired autophagic flux.(11) Increasing evidence supports that reactive oxygen species (ROS), oxidized lipoproteins (oxLDL), and endoplasmic reticulum (ER) stress activate autophagy.(12-14) We have established that OSS activates autophagy, but impairs the completion of autophagy process, leading to endothelial mitochondrial DNA damage, whereas PSS favors autophagic flux to maintain mitochondrial homeostasis.
17. Takabe W, Jen N, Ai L, Hamilton R, Khalsa B, Darbandi F, Bressler S, Barr M, Li R, Hsiai* TK. Oscillatory Shear Stress Induces Mitochondrial Superoxide Production: Implication of NADPH Oxidase and c-Jun NH2-terminal Kinase Signaling. Antioxidants & Redox Signaling. 2011 Sep 1;15(5):1379-88 PMID: 20919940
18. Rongsong Li, Nelson Jen, Lan Wu, Juhyun Lee, Karen Fang, Katherine Quigley, Katherine Lee, Sky Wang, Bill Zhou, Laurent Vergnes, Yun-Ru Chen, Zhaoping Li, Karen Reue, David Ann, Tzung Hsiai*.Disturbed Flow Induces Autophagy But Impairs Autophagic Flux to Influence Mitochondrial Homeostasis. Antioxidants & Redox Signaling. 2015 Nov 20;23(15):1207-19 (Covered page)
19. Lee J, Packard, RG, Hsiai, TK*. Blood Flow Modulation of Vascular Dynamics. Current Opinion in Lipidology. 2015 Oct;26(5):376-83
Computational Fluid Dynamics (CFD) simulations: Rupture of a thin fibrous cap in the setting of inflammation and high shear stress is considered to be one of the main mechanisms underlying mechanically unstable plaque. Partial ligation in the mid-common carotid artery induces post-stenotic disturbed flow that promotes inflammatory responses and atherogenesis. Intravascular shear stress will be measured in real-time to provide a global wall shear stress and flow characteristics around the ligation. CFD simulations will be reconstructed from pulsed-wave Doppler and IVUS to complement intravascular shear stress (ISS) signals and immunohistochemistry, revealing low shear areas for lesion formation versus high shear areas for predisposition to rupture of existing lesions as previously described (Fig. 4).
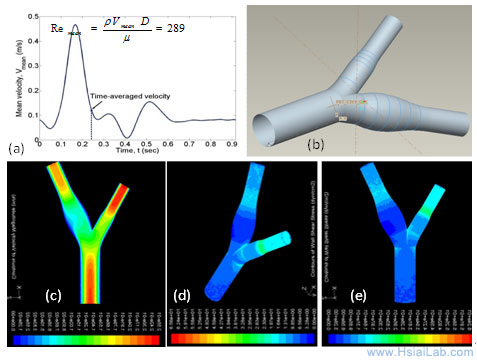
Fig. 4. A representative CFD reconstruction for the carotid artery. (a) Pulsatile flow profile derived from pulsed-wave Doppler. (b) A 3-D geometry from the mesh generation. (c) A pulsatile velocity profile. (d) - (e) Wall shear stress.
20. Ai, L. Yu, H., Paraboschi, A., Li, R., T. K. Hsiai*, Optimization of Intravascular Shear Stress Assessment. J of Biomechanics. 2009 Jun;56 (6):1755-64.
21. Ai, L., H. Yu, S. Hale, W. Dai, R. A. Kloner, T. K. Hsiai*, Real-time intravascular shear stress in rabbit aorta. IEEE Transactions on Biomedical Engineering. 2009 Jun;56(6):1755-64.
Mechanobiology and Developmental Biology
Hemodynamic forces such as shear stress are intimately linked with cardiac morphogenesis. Mutations in Notch signaling components result in congenital heart defects in humans and mice. During heart development, the myocardium differentiates into two layers, an outer compact zone and an inner trabeculated zone. The trabeculae form a network of branching outgrowths from the ventricular wall,3 and both trabeculation and compaction are essential for normal cardiac contractile function. Hemodynamic shear stress induces vascular endothelial Notch signaling, and Notch pathway mediates differentiation and proliferation of trabecular myocytes. Our multi-disciplinary approach has demonstrated that peristaltic contraction of the embryonic heart tube produces time-varying shear stress ![]() and pressure gradients
and pressure gradients ![]() across the atrioventricular (AV) canal in a zebrafish model of cardiac development. The advent of zebrafish genetic system has enabled the application of fli1 promoter to drive expression of enhanced green fluorescent protein (EGFP) in all vasculature throughout embryogenesis8 (Tg(fli1a:EGFP)y1); thereby, allowing for 3-D visualization of the moving boundary conditions (2D + time) for computational fluid dynamics (CFD) simulation. However, 4-D (3-D + time) imaging to recapitulate the endocardium throughout the cardiac cycle requires fast tissue scanning and deep axial penetration. For these reasons, we have developed multi-scale fluorescent Super-Resolution Light-Sheet Microscopy (SRLSM) to capture the 4-D endocardial trabecular network.
across the atrioventricular (AV) canal in a zebrafish model of cardiac development. The advent of zebrafish genetic system has enabled the application of fli1 promoter to drive expression of enhanced green fluorescent protein (EGFP) in all vasculature throughout embryogenesis8 (Tg(fli1a:EGFP)y1); thereby, allowing for 3-D visualization of the moving boundary conditions (2D + time) for computational fluid dynamics (CFD) simulation. However, 4-D (3-D + time) imaging to recapitulate the endocardium throughout the cardiac cycle requires fast tissue scanning and deep axial penetration. For these reasons, we have developed multi-scale fluorescent Super-Resolution Light-Sheet Microscopy (SRLSM) to capture the 4-D endocardial trabecular network.
The 2-D simulation data paves the way to expand to 3-D CFD of heart development (Fig. 5). Coupling the moving boundaries with the time-dependent LSM images will further enable us to elucidate mechanotransduction underlying spatial and temporal variations in endocardial Notch signaling and trabeculation. In collaboration with Prof. C.-C. Jay Kuo (USC), we will incorporate computational algorithms to synchronize cardiac cycle with the LSM-captured images for time-dependent reconstruction of 3-D CFD. Despite a plethora of literature linking mechanotransduction and atherosclerosis, our team effort will be the first to elucidate shear stress-activated endocardial Notch signaling and cardiac trabeculation.

Fig. 5. Moving domain simulation during zebrafish cardiac morphogenesis. At 20-30 hr post fertilization (hpf), the heart is a tube-like structure with an AV canal separating the atrium (A) and ventricle (V). (a) Atrial contraction engenders an increase in shear stress profiles through the AV canal. Velocity vectors highlight unidirectional forward flow from the contracting atrium into the ventricle through the AV canal. (b) At 40-50 hpf, the zebrafish heart begins to undergo cardiac looping. Compared to 20-30 hpf, Wall shear stress (WSS) across the AV canal is increased by ~3-fold during atrial contraction with a concomitant decrease in flow reversal during ventricular contraction. (c) At 110-120 hpf, the AV valve is distinct and left ventricle is enlarging. Complete formation of the AV valve and bulbus arteriosus results in a reduced flow reversal.
2-D moving domain CFD: We have established our 2-D simulation of endocardiac hemodynamics during cardiac development (Fig. 6). In collaboration with Prof. Alison Marsden from UCSD, we have incorporated moving-domain CFD analysis to recapitulate the changes in shear stress across the atrioventricular (AV) valves from 20 days to 120 day post fertilization (hpf). While hemodynamic shear stress is considered to be an epigenetic factor influencing vascular oxidative stress and inflammatory responses, there remains a paucity of methodologies to investigate spatial and temporal changes in endocardial shear stress during cardiac development. Thus, our innovation resides in the reconstruction of time-dependent moving endocardial boundaries and interpolation between measurements of the dynamic embryonic hearts. To reconstruct the 2-D CFD models, we have focused on the mid-plane in which blood flows through the AV canal. Recorded video was extracted frame-by-frame to track the motion of the beating hearts. Wall motion was generated by the interpolation between frames. The inlet velocity boundary condition from particle image velocity (PIV) was employed to corroborate the CFD simulation. A dramatic increase in blood velocities, wall shear stress, and heart rates developed from 20 hpf to 70 hpf (Fig. 6). Thus, we demonstrated a reasonable agreement between 2-D simulation and PIV data.
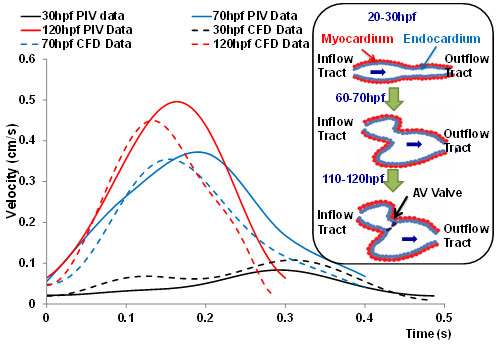
Fig. 6. CFD simulation across AV canal during cardiac development. The velocity profiles were validated by comparing with those of particle image velocimetry (PIV) via the Matlab-coded PIV tool; namely, 20-30 hpf, 60-70 hpf, and 110-120 hpf. The CFD simulation overlapped reasonably with those of PIV. Noticeable are the increase in both the blood velocities and heart rates at the late stages. The inset illustrates the schematic cardiac development. At 20-30 hpf, the heart is a tube-like structure. At about 50 hpf, the heart tube is undergoing looping. At 110-120 hpf, AV cushion develops into a AV valve.
Vorticity fields and instantaneous flow streamlines reveal laminar flow profiles occurring from early to late stages (Fig. 7). At 20-30 hpf, unsteady vortex features occur in response to atrial relaxation (Fig. 6b). At 110-120 hfp, unsteady vortices occur in both the atrium and ventricle (Fig. 7c and d). While the mechano-biological implication of disturbed or vortical flow remains to be established, trabeculation was observed during late developmental stages, supporting clinical relevance to study mechanotransduction and trabeculation.
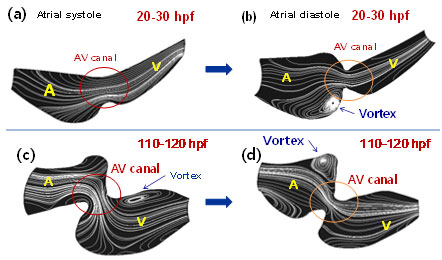
Fig. 7. Instantaneous streamlines from early to late stages. (a-b) At 20-30hpf, vortices in the morphological atrium is present in response to atrial diastole. (c-d) At 110-120 hpf, vortices are present in the ventricle during atrial systole and in the atrium during atrial diastole.
22. Juhyun Lee, Mahdi Esmaily-Moghadam, Ethan Kung, Hung Cao, Tyler Beebe, Longhou Fang, Yuri Miller, Ching-Ling Lien, Neil C. Chi, Alison L. Marsden, and Tzung K. Hsiai*. Moving Domain Computational Fluid Dynamics to Interface with An Embryonic Zebrafish Model of Cardiac Morphogenesis. PLoS ONE. 2013 Aug 23;8(8):e72924.
Fluid shear stress intimately regulates vasculogenesis and endothelial homeostasis. The canonical Wnt/β-catenin signaling pathways play an important role in differentiation and proliferation. In this study, we investigated whether shear stress activated Angiopoietin-2 (Ang-2) via the canonical Wnt signaling pathway with an implication in vascular endothelial repair. Oscillatory shear stress (OSS) up-regulated both TOPflash Wnt reporter activities and the expression of Ang-2 RNA and protein in human aortic endothelial cells (HAEC) accompanied by an increase in nuclear β-catenin intensity. Both Ang-2 and Axin-2 mRNA down-regulation was recapitulated in the heat-shock inducible transgenic Tg(hsp70l:dkk1-GFP) zebrafish embryos at 72 hours post fertilization (hpf). Ang-2 morpholino injection of Tg (kdrl:GFP) fish impaired subintestinal vessel (SIV) formation at 72hpf, which was rescued by zebrafish Ang-2 mRNA (zAng-2) co-injection (Fig. 8). Inhibition of Wnt signaling with IWR-1 also down-regulated Ang-2 and Axin-2 expression, and impaired vascular repair after tail amputation, which was rescued by zAng-2 injection.
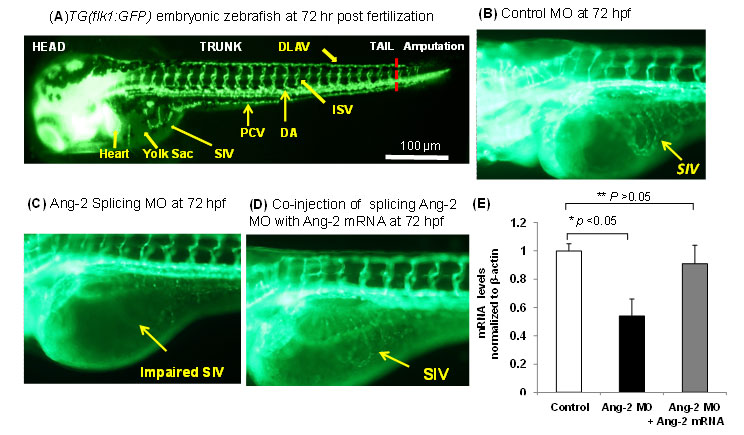
Fig. 8. Ang-2 morphant injection impaired subintestinal vein (SIV) formation in Zebrafish embryos. (A) Vasculature of transgenic zebrafish Tg (Fli-1:GFP) at 72hpf reveals subintestinal vessel (SIV), intersegmental vessel (ISV), dorsal longitudinal vein (DLAV), dorsal aorta (DA), and posterior cardinal vein (PCV). (B) Embryos injected with the control MO developed normal SIV at 72 hpf. (C) Ang-2 splicing MO injection (0.3 µm) impaired SIV formation. (D) Co-injection of Ang-2 mRNA with Ang-2 MO rescued SIV formation. (D) Ang-2 mRNA expression was significantly down-regulated in response to Ang-2 splicing MO injection at 72 hpf (*p < 0.05, n=6). Co-injection with Ang-2 mRNA (25ng) rescued Ang-2 mRNA expression (**p > 0.05, n=6).
23. Rongsong Li, Tyler Beebe, Fei Yu, Wakako Takabe, Michael Harrison, Hung Cao, Juhyun Lee, Hongbo Yang, Peidong Han, Kevin Wang, Hirohito Shimizu, Jaunian Chen, Ching-Ling Lien, Neil C. Chi, Tzung K. Hsiai*. Shear Stress-Activated Wnt-Angiopoeitin-2 Signaling Recapitulates Vascular Development and Repair in Zebrafish Embryos. Arteriosclerosis Thrombosis, and Vascular Biology. 2014;34:2268-2275.
Hemodynamic shear forces are intimately linked with cardiac development, during which trabeculae form a network of branching outgrowths from the myocardium. Mutations in Notch signaling result in hypotrabeculation. We assessed whether shear stress modulated trabeculation to influence contractile function. We acquired 4-D (3-D+time) images with light-sheets by Selective Plane Illumination Microscopy (SPIM) for rapid scanning and deep axial penetration during zebrafish morphogenesis. Reducing blood viscosity via gata1a morpholino oligonucleotides (MO) reduced shear stress, resulting in down-regulation of Notch signaling and attenuation of trabeculation. Arresting cardiomyocyte contraction via tnnt2a MO or inhibiting atrial contraction via the weak atriumm58 (wea) mutants also reduced shear stress to down-regulate Notch signaling and trabeculation.
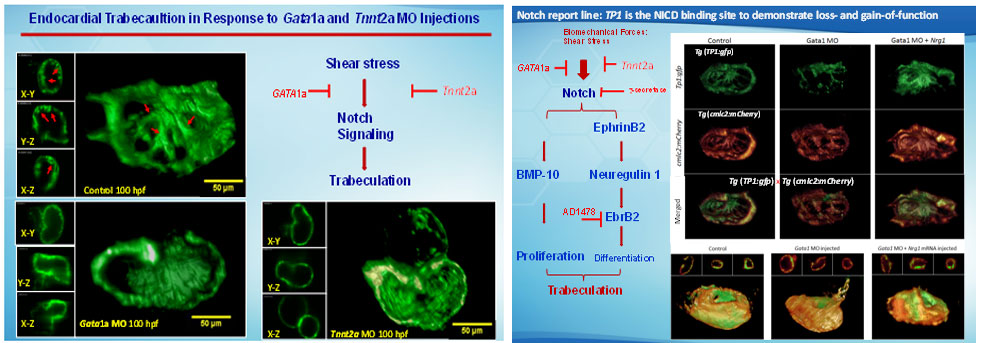
The dynamics of 3D cardiac architecture in response to genetic manipulations of the Tg(cmlc:gfp) zebrafish system. (A 3D Cartesian coordinate system provides the orientation of the ventricle. The ventricular inflow tract from the atrium relates to the outflow tract from the 3D ventricle. Trabecular network developed in the ventricle at 75 hpf in the WT (control). Trabeculae formed a prominent sponge-like structure at 100 hpf in the WT. gata1a MO microinjection at the 1- to 4-cell stage attenuated trabeculation at 75 hpf. gata1a MO–treated fish delayed trabecular formation at 100 hpf. Coinjection of Nrg1 mRNA promoted trabeculation at 75 hpf. Coinjection of Nrg1 mRNA nearly restored trabecular network at 100 hpf. Trabeculation was absent in the wea mutants with a small ventricle. Injection of Nrg1 mRNA into the wea mutants promoted trabecular formation. The tnnt2a MO microinjection inhibited trabeculation at L) The clo mutants developed nontrabeculated endocardium at 100 hpf. Red arrows indicate trabecular ridges. Scale bars: 50 μm. Original magnification, ×10.

Regional strain measurements. (A) Trabecular (yellow lines) and non-trabecular (red lines) regions from controls as well as their combined myocardial segment (purple lines) are illustrated at end-systole and end-diastole. (B) ErbB signlaing inhibitor (AG1478) treatment inhibited trabeculation. Non-trabeculated myocardium (dark blue lines) and the combined myocardial segment (sky blue line) are illustrated at end-systole and end-diastole. (C) Segmental strain curves from trabeculated control regions (red lines), non-trabeculated control regions (yellow lines) and AG1478–treated non-trabeculated regions (dark blue lines) are illustrated. (D) Comparing the combined strain between controls (purple) and AG1478 treatment (sky blue) illustrates the a decrease in contractility in response to AG1478.
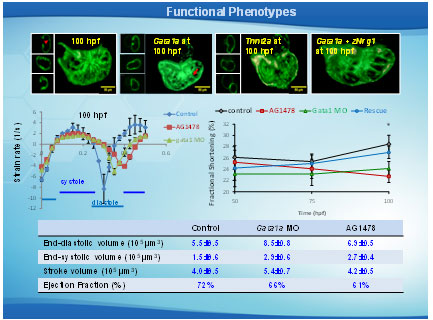
Effects of trabeculation on strain and fractional shortening. Strains were depicted at (A) 50 hpf, (B) 75 hpf, and (C) 100 hpf in response to AG1478 treatment and gata1a MO injection. (C) Significant decrease and delay in strain were observed in response to AG1478 (red) and gata1a MO (green) as compared with the WT (control, black) at 100 hpf. Coinjection of Nrg1 mRNA (blue) with gata1a MO improved strain at 100 hpf. (D) AG1478 and gata1a MO significantly decreased fractional shortening at 100 hpf. Coinjection of Nrg1 mRNA with gata1a MO improved FS. (E and F) Integrating 4D synchronization algorithm with SPIM revealed that AG1478 and gata1a MO groups developed increased ESV and EDV.
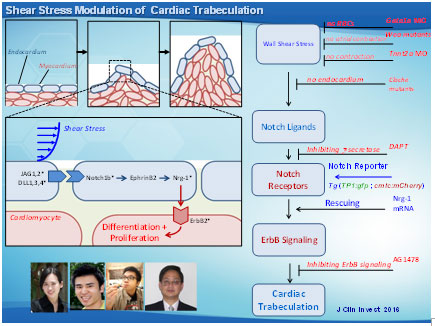
Shear stress activation of Notch signaling to promote trabeculation. (A) Shear stress modulates trabecular formation in the myocardium. The inset reveals the activated Notch signaling in the endocardial cells (blue). Asterisks indicate tested genes. (B) A host of genetic manipulations elucidates the mechanotransduction of Notch signaling pathway underlying shear stress and initiation of trabeculation. (C) Nrg1 mRNA injection rescues trabeculation, which, in turn, restores cardiac contractile function. The gain of function in contractility generates hemodynamic shear forces to activate Notch signaling.
Overall, we provide the first evidence that hemodynamic shear stress modulates cardiac trabeculation via Notch signaling. Integration of 4-D light-sheet imaging with the genetically engineered zebrafish system provides a dynamic model to establish a fundamental direction at the interface of biomechanical forces and cardiac development. We demonstrate the fundamental significance of blood flow underlying cardiac architecture and function relevant to non-compaction cardiomyopathy in congenital heart disease.
24. Juhyun Lee, Peng Fei, Nelson Jen, Tyler Beebe, Debra Yelon, Neil Chi, C. C. Cuo, Chih-Ming Ho, Rongsong Li, Tzung Hsiai*. 4-D Light-Sheets to Elucidate Shear Stress Modulation of Cardiac Trabeculation. Journal of Clinical Investigation . 2016 May 2:126(5):1679-90
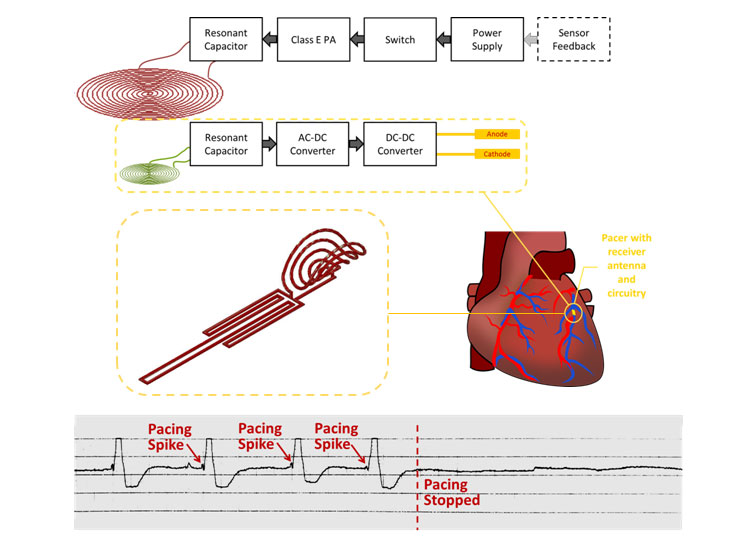
Despite great advancements in implantable cardiac pacemaker technology, complications from pacemaker leads continue to compromise nearly 10% of all implants. This has motivated significant research for the development of leadless devices. These include battery-based systems, power harvesting devices, and stimulators wirelessly powered through radiofrequency radiation. Our research is focused on the development of an inductive power transfer system with a remote stimulation control system. Our proposed system is capable of significantly improving power efficiency and reducing tissue energy absorption in a highly asymmetrical system in which the pacer is small enough to be intravascularly deployed and implanted with a stent-like fixation mechanism.
Mechanically Unstable Plaque

Early detection of metabolically and mechanically unstable plaque during angioplasty for patient-specific intervention.
More than 18 million North Americans have atherosclerotic disease, and both morbidity and mortality remain appreciable in the Western world. In the U.S., the prevalence of overt coronary artery disease is about 7 million with up to 2 million procedures performed annually. Despite the advent of computed tomographic (CT) angiography, high resolution MRI, intravascular ultrasound (IVUS), near-infrared fluorescence (NIRF), time-resolved laser-induced fluorescence spectroscopy, and other imaging modalities, identifying metabolically active lesions prone to rupture remains an unmet clinical need. Hence, enhancing the characterization of active lipid- and foam cell-rich plaques in terms of electrochemical impedance spectroscopy (EIS) may offer a translational implication for patient-specific intervention.
Plaque rupture remains the hallmark of acute coronary syndromes. Encouraging results from our Cardiovascular Engineering Research Group has demonstrated that integration of intravascular shear stress (ISS) and endoluminal electrochemical impedance spectroscopy distinguishes pre-atherogenic lesions associated with oxidative stress Specifically, vessel walls harboring oxidized low density lipoprotein (oxLDL) exhibit distinct electrochemical impedance spectroscopy (EIS) signals, and that monocytes and oxLDL together destabilize calcific vascular nodules via induction of matrix metalloproteinase (MMP). In this context, we have developed an electrochemical strategy to characterize the metabolically active, albeit non-obstructive, lesions during diagnostic angiogram or primary coronary intervention. We have established that active lipids and macrophages caused distinct electrochemical properties in the vessel wall that can be measured by electrochemical impedance spectroscopy (EIS) to identify metabolically active plaque.
Our interdisciplinary efforts, including UCLA Atherosclerosis Research Unit (Fogelman, Navab, Reddy), UCLA Vascular Calcification (Demer and Tintut), UCLA Medical Imaging Bioinformatics (Bui), USC NIH Ultrasonic Transducer Resource Center (Shung), and UCLA and Caltech Micro- and Nano-Sensor Technology (Hsiai, Tai), UCLA/GLA Interventional Cardiology (Aksoy, Ebrahimi, Suh, Tobis), have provided new insights into the electrochemical strategy to characterize metabolically active plaque prone to rupture for patient-specific intervention.
Team Science
Rapid Liposome Mimicking SARS-CoV-2 to Elucidate Thrombosis in Endothelialized Microfluidic Chip

Angela Lai, Sandro Satta, Susana Cavallero, Cayden Williamson, Dino Di Carlo, Tzung Hsiai.
NIH T32 Scholar for Integrating AI and ML to study cardiometabolic dysfunction
The UCLA/Caltech T32 Training Program is seeking candidates with PhD or equivalent training in biological, bioengineering, or quantitative science for our NIH-funded T32 Post-Doctoral Training opportunity. The UCLA/Caltech iTEAM program provides co-mentorhsip essential for bridging AI and ML with human genetics and cardiometabolic disorders. The candidates will be able to develop deep learning algorithms that address the missing data and to elucidate cardiac diastolic heart failure. The candidates will have opportunity to engage in our Caltech/UCLA T32 symposium for scientific collaboration and connection with Amazon Science Hub for Humanity and Artificial Intelligence.
The iTEAM program is dedicated to training the next generation of engineers and biophysical and biomedical scientists. In this pursuit, our program is committed to promoting diversity, inclusion, and equity, and we welcome applicants with disabilities, from low SES backgrounds and from racial and ethnic groups under-represented in the biomedical and quantitative sciences.
NIH - NATIONAL HEART, LUNG, AND BLOOD INSTITUTE
INSTITUTIONAL NATIONAL RESEARCH SERVICE AWARD – T32 HL144449
UCLA/Caltech Integrated Cardiometabolic Medicine for Bioengineers (iCMB)
The University of California at Los Angeles (UCLA) and the California Institute of Technology (Caltech) are partnering to provide a 2-year, structured curriculum for training post-doctoral engineers and biophysical or biomedical scientists to prepare them for leadership roles in academia and industry. The goal of this T32 program is to strengthen individualized training in 1) advanced sensing or 2) imaging coupled with machine learning usually in the context of addressing metabolic disease with an understanding of how to improve access to quality of care.
The UCLA/Caltech iCMB program employs a co-mentorship model, and each post-doctoral scholar will have a primary mentor from cardiometabolic disease and a co-mentor from enabling technologies and/or industry. Post-doctoral scholars will work with their mentors to identify and track individualized research and training goals and milestones for the 2-year period.
The UCLA/Caltech iCMB program is dedicated to training the next generation of engineers and biophysical and biomedical scientists. In this pursuit, our program is committed to promoting diversity, inclusion, and equity, and we welcome applicants with disabilities, from low SES backgrounds and from racial and ethnic groups under-represented in the biomedical sciences.
NIH - NATIONAL INSTITUTE OF BIOMEDICAL IMAGING AND BIOENGINEERING
INSTITUTIONAL NATIONAL RESEARCH SERVICE AWARD – T32EB023858
Caltech/UCLA Integrated Theronastic Engineering to Advance Metabolic Systems (iTEAM)
The Caltech/UCLA integrated Theranostic Engineering to Advance Metabolic Medicine (iTEAM) Program represents a new paradigm to partner with industry leaders (Amgen, Johnson & Johnson, Medtronic, and Edwards Lifesciences) for internship, mentorship, and leadership programs. Each iTEAM scholar will have co-mentorships: a primary mentor from enabling technologies and a secondary from cardiometabolic medicine and/or industry.
The Caltech/UCLA iTEAM program employs a co-mentorship model, and each post-doctoral scholar will have a primary mentor from enabling technologies and a co-mentor from metabolic medicine and/or industry. Post-doctoral scholars will work with their mentors to identify and track individualized research and training goals and milestones for the 2-year period.
The Caltech/UCLA iTEAM program is dedicated to training the next generation of engineers and biophysical and biomedical scientists. In this pursuit, our program is committed to promoting diversity, inclusion, and equity, and we welcome applicants with disabilities, from low SES backgrounds and from racial and ethnic groups under-represented in the biomedical sciences.
UCLA Outreach for STEM PROGRAMS
Students, teachers, UCLA undergraduates, graduates, project scientist, physician, faculty, and administrators were taking the final photo.

Caltech/UCLA Joint Meeting at The Caltech Annenberg Center for Information Science Technology
Attendants: Professor YC. Tai (Caltech). Linda L. Demer (UCLA), Parinaz Abiri (Caltech/UCLA MSTP student), YUan Luo (Caltech Post-Doc), Packard Wray (Caltech PhD student), Lei Li (Caltech Post-Doc), Rene Packard (UCLA), Kyung Baek (UCLA), Juhyun Lee (UCLA), Yichen Ding (UCLA), Chih-Chiang Chang (UCLA), Tzung Hsiai (UCLA)

Academia-Industry Partnership at the 18th UC System-wide Bioengineering Symposium at UCLA Luskin Conference Center in June, 2017
Speaker: Linda Narhi, PhD, Scientific Executive Director, Process Development, Amgen Inc.

11th Interntional Symposium on BioMechanics in Vascular Biology and Cardiovascular Disease
Hanjoong Jo (Emory/Geogia Tech), John Oshinski (Emory), Robert Taylor (Emory), Ajit Yoganathan (Georgia Tech), Paul Evans (University of Sheffield, UK), Frank Gijsen (Erasmus Medical Center), Kim van der Heiden (Erasmus Medical Center), Jolanda Wentzel (Erasmus Medical Center), John Tarbell(The City College of New York), Tzung Hsiai (UCLA)

Brainstorming session with clinicians, bioengineers, and scientists at UCLA Ronald Reagon Medical Center.
